I’m not sure if this is a Chinese saying, but once, I heard that the more symmetrical a face between the left and right sides, the more attractive it is. This occurred to me again when I was doing my eyebrows last night and accidentally trimmed a little too much off my left eyebrow. I’m going to be an unattractive woman for the next seven days.
Anyway, mirrors can be found all over nature. Our left and right ears are mirror images of each other. Leaves are often symmetrical between the left and right sides. In fact, mirrors in nature exist at a molecular level. When a molecule is such that there exists another one that would be a mirror image of itself, the pair is known as stereo isomers.
 |
| A pair of stereo isomers |
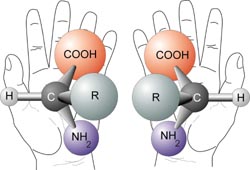
I’m going to introduce this scientific term here: Chiral. It’s a beautiful little word and it won’t bite. Say it out loud. The word, chiral, is an adjective used to describe a molecule that has a stereo isomer. Chemists have come up with a way to name the stereo isomers so that we don’t have to keep saying ‘the molecule and its mirror image.’ Based on a set of criteria, one of them is named ‘R’ and the other ‘S’.
R and S isomers are like our left and right hands – essentially identical, until you put them one on top of each other, both palms facing up. Our hands are chiral. Humans are chiral. The receptors in our bodies that bind to drugs are chiral. So if we take drugs that are chiral, the R and S will affect us differently.
R-thalidomide was the good one that helped pregnant women with morning sickness. S-thalidomide, however, inserted itself into DNA and stopped fetal limbs from developing. The drug was sold as an equal mixture of both – at that time, separation of the R and S versions of a molecule was not well understood.
How can the R and S versions of a molecule be separated? They have the same weight, they melt and boil at the same temperatures, they dissolve in the same solvents – R and S have the same response to practically everything, until a chiral environment is created.
In the simplest terms, a chiral environment is a solution containing either the R or S version of a molecule. Chiral molecules that occur in nature, such as amino acids and sugars, are found in only one form – either the R or the S. A very commonly used one is tartaric acid, found in grapes and bananas. Chemists extracted the pure isomer of these molecules from nature and used them to create chiral environments by dissolving the pure isomer. That was how the first separations of R and S molecules were performed.
For instance, a chemist would first dissolve a pure R-compound in a solvent to make a solution. The solution now consists of a chiral environment. He/she would then place a mixture of R and S isomers that he/she wants to separate in the solution. After some time, the R isomer, for example, would appear as crystals while the S remained in solution with the pure R-compound.
That’s not very efficient if only the R or the S isomer is useful to the chemist, because then the other isomer would just be discarded. In a typical drug synthesis, there are some seven to thirty steps and if, in each step, half of the product has to be discarded in this manner, so many resources would be wasted just to make this one drug.
Chemists have since come up with many more solutions to the problem. The most efficient, and by far the best, methods work to force the product of a reaction to be either the R or S isomer. So instead of producing an equal mix of R and S, and then separating them, chemists can now control chemical reactions to produce only the isomer that they want. This branch of synthetic methods is known as asymmetric synthesis and the 2001 Nobel Prize in Chemistry was awarded to three chemists, Barry Sharpless, Ryoji Noyori and William Knowles, for their contributions in asymmetric synthesis.
Now, it is known that thalidomide, once in the human body, can flip between the R and S versions in the human body. So, even if pure R-thalidomide was administered, it will revert to an equal mixture of R- and S-thalidomide. In short, thalidomide should not even have been a legal drug for humans. While our reflections in the mirror act exactly as we do, the same certainly does not hold true for molecules.